It has long been known by any close FDA-watcher that the Food and Drug Administration has to balance between pharmaceutical scarcity and safety. But with drug shortages becoming a regular occurrence since the baby formula fiasco last year, the FDA has now come down on the side of placing the importance of scarcity over the importance of drug safety. That means the typical American medical patient that needs drugs that are in short supply will now face a terrible choice: take drugs that are not tested by the FDA for safety standards or take the ones imported from uninspected India and China labs instead.
The FDA is considering allowing for the temporary importation of chemotherapy drugs from overseas manufacturers that are “not currently approved to distribute in the United States”, an agency spokesperson told CNBC.
The FDA did not say which manufacturers would be potential candidates for permitting temporary importation of those drugs until approved manufacturers are able to meet patients’ needs. But, “in these cases, we very carefully assess the overseas product for quality, making sure that it’s safe for U.S. patients,” the spokesperson said. How they can do this without inspection is unknown, but it is likely to mean the product will come from FDA labs that have at least once been inspected by FDA officials. As it is, since the pandemic, many of these labs have gone without official inspections since 2020. There have been numerous recalls of drugs from Indian labs over the last two years, almost all of them voluntary recalls. It is unknown how many drugs should have been recalled due to impurities, but were not as most recalls are done on the decision of the manufacturer and not the FDA.
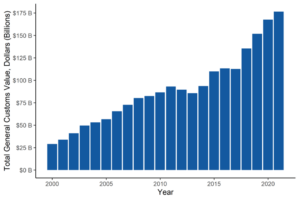
U.S. pharmaceutical imports from 2000 to 2021 have risen six-fold. Yet, shortages persist. Incentives are needed to produce the daily-use critical drugs that are consistently in short supply.
Drug Shortages Everywhere
The American news diet is not short on stories of drug shortages.
In March, The New York Times called shortages of everything from oncology medications used for cancer patients, to basic pneumonia-fighting drugs like amoxicillin a “national security threat”. Such language is often seen as being the best way to frame an issue in order to garner the attention of policymakers on Capitol Hill. CPA’s senior economist Andrew Heritage did a report on this in January.
Drug shortages hit an all-time high this winter, according to a report by the Senate Homeland Security and Government Affairs Committee. Last year saw a record five-year high of 295 active drug shortages.
The FDA said 14 cancer drugs are now in short supply. “The oncology shortage is especially critical,” FDA Commissioner Dr. Robert Califf told NBC News on May 26. “I’m a former intensivist doctor and I’m very aware of the consequences if you can’t get needed chemotherapy.”
One of the drugs, capecitabine, often sold under the brand name Xeloda among others, is used to treat breast cancer, gastric cancer and colorectal cancer. According to the FDA’s website, the only U.S. source of that drug is California-based Genentech, and they only have it in 500 milligram tablets. The other sources are Dr. Reddys and MSN Laboratories in India, and Teva Pharmaceuticals of Israel. The U.S. is clearly the world’s biggest consumer, but has no one locally to make it for the market.
Dr. Lucio Gordan, a medical oncologist and president of Florida Cancer Specialists and Research Institute, a network of cancer clinics, told NBC News that his clinic was completely out of the drug for nearly two weeks.
“I’ve been doing this for 20-plus years. This is the worst I’ve ever seen,” he said.
India and China are increasingly the leading U.S. source for generic pharmaceuticals, which account for 90% of all prescriptions written in the U.S. Federal policy can turn around the offshoring of pharmaceutical production and our reliance on India and China. Tax credits for domestic production and other policies are under consideration in Congress and the Biden administration. The U.S. largely manufactures branded drugs that are highly profitable while generic drugs Americans rely on for standard prescriptions or things like injectable antibiotics are predominately sourced from poor countries where production costs are low and regulatory standards are sometimes nonexistent. – “Skyrocketing Pharmaceutical Imports to the U.S. Endanger National Security” by Andrew Heritage, Senior Economist, Coalition for a Prosperous America, January 2023.
In early May, the House Energy and Commerce Subcommittee on Oversight and Investigations held a hearing on drug shortages. During his opening remarks on the day, Chairman Morgan Griffith (R-VA) said, “The typical generic drug has just two manufacturing facilities here. We currently do not fully utilize the factories we have. We only use about half of our current generic manufacturing capacity. We need an FDA that prioritizes applications from U.S. manufacturers and gives companies the flexibility to address shortages with resources based here.”
Rep. Cathy Rodgers (R-WA) said the FDA is an ineffective partner in combating drug shortages. “The FDA last testified that around 80% of drug ingredients and 60% of finished dosage facilities are overseas. These countries limit our foreign drug inspection program’s ability to operate adequately. It’s an enormous problem if we cannot properly inspect the quality of ingredients in common drugs Americans rely on. This raises concerns over drug quality,” she said.
But the FDA is saying that drug shortages mean uninspected drugs may be needed to fill the gaps, gaps the U.S. manufacturer is unable to fill at this time.
In the waning months of the Trump Administration, the FDA was tasked with compiling a list of critical drugs in short supply. While that list has been produced, no legislation has been voted on by the current government to provide any form of incentive to labs producing these perennially short-supply, critical drugs in the U.S.
In an op-ed in The Washington Times on May 28, Sen. Marco Rubio (R-FL) laid some of the blame on Washington for the malaise. “Our leaders spent decades encouraging the industry to consolidate and move offshore. They told us that this was an unavoidable outcome of globalization and that the jobs lost by Americans would be more than offset by greater economic efficiency and lower consumer costs. They were wrong,” Rubio said. “We desperately need to restore a robust generic drug manufacturing market on American soil.”
To Fight Drug Shortages FDA Has an Idea: Import More Inspection-Free Drugs
It has long been known by any close FDA-watcher that the Food and Drug Administration has to balance between pharmaceutical scarcity and safety. But with drug shortages becoming a regular occurrence since the baby formula fiasco last year, the FDA has now come down on the side of placing the importance of scarcity over the importance of drug safety. That means the typical American medical patient that needs drugs that are in short supply will now face a terrible choice: take drugs that are not tested by the FDA for safety standards or take the ones imported from uninspected India and China labs instead.
The FDA is considering allowing for the temporary importation of chemotherapy drugs from overseas manufacturers that are “not currently approved to distribute in the United States”, an agency spokesperson told CNBC.
The FDA did not say which manufacturers would be potential candidates for permitting temporary importation of those drugs until approved manufacturers are able to meet patients’ needs. But, “in these cases, we very carefully assess the overseas product for quality, making sure that it’s safe for U.S. patients,” the spokesperson said. How they can do this without inspection is unknown, but it is likely to mean the product will come from FDA labs that have at least once been inspected by FDA officials. As it is, since the pandemic, many of these labs have gone without official inspections since 2020. There have been numerous recalls of drugs from Indian labs over the last two years, almost all of them voluntary recalls. It is unknown how many drugs should have been recalled due to impurities, but were not as most recalls are done on the decision of the manufacturer and not the FDA.
U.S. pharmaceutical imports from 2000 to 2021 have risen six-fold. Yet, shortages persist. Incentives are needed to produce the daily-use critical drugs that are consistently in short supply.
Drug Shortages Everywhere
The American news diet is not short on stories of drug shortages.
In March, The New York Times called shortages of everything from oncology medications used for cancer patients, to basic pneumonia-fighting drugs like amoxicillin a “national security threat”. Such language is often seen as being the best way to frame an issue in order to garner the attention of policymakers on Capitol Hill. CPA’s senior economist Andrew Heritage did a report on this in January.
Drug shortages hit an all-time high this winter, according to a report by the Senate Homeland Security and Government Affairs Committee. Last year saw a record five-year high of 295 active drug shortages.
The FDA said 14 cancer drugs are now in short supply. “The oncology shortage is especially critical,” FDA Commissioner Dr. Robert Califf told NBC News on May 26. “I’m a former intensivist doctor and I’m very aware of the consequences if you can’t get needed chemotherapy.”
One of the drugs, capecitabine, often sold under the brand name Xeloda among others, is used to treat breast cancer, gastric cancer and colorectal cancer. According to the FDA’s website, the only U.S. source of that drug is California-based Genentech, and they only have it in 500 milligram tablets. The other sources are Dr. Reddys and MSN Laboratories in India, and Teva Pharmaceuticals of Israel. The U.S. is clearly the world’s biggest consumer, but has no one locally to make it for the market.
Dr. Lucio Gordan, a medical oncologist and president of Florida Cancer Specialists and Research Institute, a network of cancer clinics, told NBC News that his clinic was completely out of the drug for nearly two weeks.
“I’ve been doing this for 20-plus years. This is the worst I’ve ever seen,” he said.
In early May, the House Energy and Commerce Subcommittee on Oversight and Investigations held a hearing on drug shortages. During his opening remarks on the day, Chairman Morgan Griffith (R-VA) said, “The typical generic drug has just two manufacturing facilities here. We currently do not fully utilize the factories we have. We only use about half of our current generic manufacturing capacity. We need an FDA that prioritizes applications from U.S. manufacturers and gives companies the flexibility to address shortages with resources based here.”
Rep. Cathy Rodgers (R-WA) said the FDA is an ineffective partner in combating drug shortages. “The FDA last testified that around 80% of drug ingredients and 60% of finished dosage facilities are overseas. These countries limit our foreign drug inspection program’s ability to operate adequately. It’s an enormous problem if we cannot properly inspect the quality of ingredients in common drugs Americans rely on. This raises concerns over drug quality,” she said.
But the FDA is saying that drug shortages mean uninspected drugs may be needed to fill the gaps, gaps the U.S. manufacturer is unable to fill at this time.
In the waning months of the Trump Administration, the FDA was tasked with compiling a list of critical drugs in short supply. While that list has been produced, no legislation has been voted on by the current government to provide any form of incentive to labs producing these perennially short-supply, critical drugs in the U.S.
In an op-ed in The Washington Times on May 28, Sen. Marco Rubio (R-FL) laid some of the blame on Washington for the malaise. “Our leaders spent decades encouraging the industry to consolidate and move offshore. They told us that this was an unavoidable outcome of globalization and that the jobs lost by Americans would be more than offset by greater economic efficiency and lower consumer costs. They were wrong,” Rubio said. “We desperately need to restore a robust generic drug manufacturing market on American soil.”
MADE IN AMERICA.
CPA is the leading national, bipartisan organization exclusively representing domestic producers and workers across many industries and sectors of the U.S. economy.
TRENDING
CPA: Liberty Steel Closures Highlight Urgent Need to Address Mexico’s Violations and Steel Import Surge
CPA Applauds Chairman Jason Smith’s Reappointment to Lead House Ways and Means Committee
Senator Blackburn and Ossoff’s De Minimis Bill is Seriously Flawed
JQI Dips Due to Declining Wages in Several Sectors as November Jobs Total Bounces Back from Low October Level
What Are Trump’s Plans For Solar in the Inflation Reduction Act?
The latest CPA news and updates, delivered every Friday.
WATCH: WE ARE CPA
Get the latest in CPA news, industry analysis, opinion, and updates from Team CPA.
CHECK OUT THE NEWSROOM ➔