As 2022 was winding down, two trade topics became front and center. Both were connected: supply chain risk, and reshoring. Everyone blamed the difficulties on pandemic health restrictions. Looking at both healthcare and the supply chain woes that the pandemic laid bare, a group of pharmaceutical companies in Europe discussed reshoring at an event run by Scrip, a pharmaceutical industry news, and information firm, in November 2022. Reshoring sounds like a good idea. But most companies scratched their head as to how, and all said costs would be too high. Given that excuse alone, it appears the global, European pharmaceutical industry is perfectly happy relying on India and China for its core generics, while it will hope it can make big money on blockbuster drugs at a handful of labs back home.
 Six panelists addressed the main challenges to reshoring active pharmaceutical ingredients (API), and whether or not reshoring was better than diversifying supply chains out of India and China. Regulation was also an issue, whereas the big pharma companies in the U.S. for example often run afoul of the Food and Drug Administration and/or the Environmental Protection Agency and get hefty fines. While these fines are often a pittance to the overall net income of these companies, it is easier for them to avoid regulatory risk by going to countries where regulatory risks are lower.
Six panelists addressed the main challenges to reshoring active pharmaceutical ingredients (API), and whether or not reshoring was better than diversifying supply chains out of India and China. Regulation was also an issue, whereas the big pharma companies in the U.S. for example often run afoul of the Food and Drug Administration and/or the Environmental Protection Agency and get hefty fines. While these fines are often a pittance to the overall net income of these companies, it is easier for them to avoid regulatory risk by going to countries where regulatory risks are lower.
Even when companies toyed with the idea of bringing manufacturing back to Europe, the move was always described as a painstaking, long-winded process.
“You can’t move manufacturing overnight, or even in a year,” said Ashu Tandon, chief commercial officer for Syngene International, which does all of its manufacturing in India. Tandon was part of the panel.
“With the regulations that are in place on the commercial end, we are pigeonholed into doing things a certain way,” said Jim Fries, CEO of Rx 360, an international pharmaceutical supply chain consortium formed in 2009 out of Philadelphia. “As an industry, how much hard work do we want to put into making potential best-practice changes…it’s easier to just go back to the way we were doing things,” he said.
The question of environmental sustainability and outsourcing pollution to Asia also came up.
“Why should we be shipping around the world constantly,” said Andrea Cusack, CEO of Leon Nanodrugs in Germany.
Despite those obstacles, some bigger than others, those who do want to localize more of their labs – especially for generic drugs and medicines deemed critical by the FDA – each will end up making their own risk/reward calculus. Manufacturing in the West will always be more costly than in Asia. Panelists agreed that companies first need to find out whether their buyers – patients, retail pharmacies, medical centers and the government – are “willing to pay a premium or whether public payers would be willing to subsidize drug production at a higher cost.”
Such subsidies exist in China, for instance. India’s Department of Science and Technology provides grants and the federal government offers investor-friendly policies (tax incentives and the like). The Department of Biotechnology is also a big funder, plus India has increased its Special Economic Zones for drug makers since 2011 and has roughly 20 pharmaceutical parks set up in these zones throughout the key states producing generic drugs for the U.S. Moreover, India’s Biotechnology Industry Research Assistance Council helps venture capital firms and has its own fund of funds that invests seed capital in pharma start-ups.
Even though India is really the biggest producer of generics in terms of revenue, China is gaining and many of the drugs India makes are dependent on China for the organic compounds and chemical starting materials used in making the drug in the first place. But it is China that garners Washington’s attention most and in September of last year, President Biden signed an Executive Order that merely outlined the goals of how to expand America’s domestic pharma footprint.
“We risk falling behind,” White House officials told the WSJ on September 12, 2022.
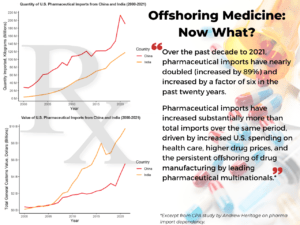 ***See CPA latest econ team report titled “Skyrocketing Pharmaceutical Imports a Threat to National Security”, by senior economist Andrew Heritage.***
***See CPA latest econ team report titled “Skyrocketing Pharmaceutical Imports a Threat to National Security”, by senior economist Andrew Heritage.***
Rosemary Gibson, author of the quintessential book on America’s dependence on foreign drugs, “China Rx: Exposing the Risks of America’s Dependence on China for Medicine”, and Chairwoman of CPA’s Healthcare Committee, says global pharma is throwing in the towel immediately if they are going to focus on price.
“U.S. manufacturers cannot compete against foreign companies that are subsidized by their governments and deploy a strategy to undercut on price to drive out U.S. producers,” Gibson said. Multibillion-dollar middlemen can take a huge share of the price paid by consumers for generic medicine. Take out the middlemen and consumers will benefit from lower-cost drugs.”
Europe may be a wee bit ahead of the U.S. on this issue. It has become more dire there, in fact, due to energy policy that increased the cost of electricity, forcing some labs to say they might have to cut production, according to a Reuters report.
In 2021, the European Commission created the Health Emergency Preparedness and Response Authority during the height of Covid supply chain problems. But a year later and the industry is still having problems.
In the Trump years, the U.S. Biomedical Advanced Research and Development Authority (BARDA) of the Department of Health and Human Services awarded four companies, including generics lab Phlow Corporation, up to $812 million. The award included a four-year base award of $354 million with an additional $458 million included as potential options for long-term investment in critical medicines.
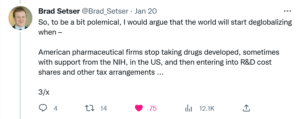 Writing on his Twitter account on January 20, former senior advisor to the United States Trade Representative from 2021 to 2022, Brad Setser, highlighted the importance of pharmaceuticals to the U.S. trade balance.
Writing on his Twitter account on January 20, former senior advisor to the United States Trade Representative from 2021 to 2022, Brad Setser, highlighted the importance of pharmaceuticals to the U.S. trade balance.
Month over month, pharmaceutical imports from India, China, and Europe, remain a top three source of our trade deficit.
One action that can reverse the trend Setser talks about above, and reshore some generic drug manufacturing is for the federal government to provide tax credits to American-owned and operated domestic companies that invest in advanced manufacturing of APIs, Gibson said.
European Pharma Considers Reshoring from India, China. Where’s the US?
As 2022 was winding down, two trade topics became front and center. Both were connected: supply chain risk, and reshoring. Everyone blamed the difficulties on pandemic health restrictions. Looking at both healthcare and the supply chain woes that the pandemic laid bare, a group of pharmaceutical companies in Europe discussed reshoring at an event run by Scrip, a pharmaceutical industry news, and information firm, in November 2022. Reshoring sounds like a good idea. But most companies scratched their head as to how, and all said costs would be too high. Given that excuse alone, it appears the global, European pharmaceutical industry is perfectly happy relying on India and China for its core generics, while it will hope it can make big money on blockbuster drugs at a handful of labs back home.
Even when companies toyed with the idea of bringing manufacturing back to Europe, the move was always described as a painstaking, long-winded process.
“You can’t move manufacturing overnight, or even in a year,” said Ashu Tandon, chief commercial officer for Syngene International, which does all of its manufacturing in India. Tandon was part of the panel.
“With the regulations that are in place on the commercial end, we are pigeonholed into doing things a certain way,” said Jim Fries, CEO of Rx 360, an international pharmaceutical supply chain consortium formed in 2009 out of Philadelphia. “As an industry, how much hard work do we want to put into making potential best-practice changes…it’s easier to just go back to the way we were doing things,” he said.
The question of environmental sustainability and outsourcing pollution to Asia also came up.
“Why should we be shipping around the world constantly,” said Andrea Cusack, CEO of Leon Nanodrugs in Germany.
Such subsidies exist in China, for instance. India’s Department of Science and Technology provides grants and the federal government offers investor-friendly policies (tax incentives and the like). The Department of Biotechnology is also a big funder, plus India has increased its Special Economic Zones for drug makers since 2011 and has roughly 20 pharmaceutical parks set up in these zones throughout the key states producing generic drugs for the U.S. Moreover, India’s Biotechnology Industry Research Assistance Council helps venture capital firms and has its own fund of funds that invests seed capital in pharma start-ups.
Even though India is really the biggest producer of generics in terms of revenue, China is gaining and many of the drugs India makes are dependent on China for the organic compounds and chemical starting materials used in making the drug in the first place. But it is China that garners Washington’s attention most and in September of last year, President Biden signed an Executive Order that merely outlined the goals of how to expand America’s domestic pharma footprint.
“We risk falling behind,” White House officials told the WSJ on September 12, 2022.
Rosemary Gibson, author of the quintessential book on America’s dependence on foreign drugs, “China Rx: Exposing the Risks of America’s Dependence on China for Medicine”, and Chairwoman of CPA’s Healthcare Committee, says global pharma is throwing in the towel immediately if they are going to focus on price.
“U.S. manufacturers cannot compete against foreign companies that are subsidized by their governments and deploy a strategy to undercut on price to drive out U.S. producers,” Gibson said. Multibillion-dollar middlemen can take a huge share of the price paid by consumers for generic medicine. Take out the middlemen and consumers will benefit from lower-cost drugs.”
Europe may be a wee bit ahead of the U.S. on this issue. It has become more dire there, in fact, due to energy policy that increased the cost of electricity, forcing some labs to say they might have to cut production, according to a Reuters report.
In 2021, the European Commission created the Health Emergency Preparedness and Response Authority during the height of Covid supply chain problems. But a year later and the industry is still having problems.
In the Trump years, the U.S. Biomedical Advanced Research and Development Authority (BARDA) of the Department of Health and Human Services awarded four companies, including generics lab Phlow Corporation, up to $812 million. The award included a four-year base award of $354 million with an additional $458 million included as potential options for long-term investment in critical medicines.
Month over month, pharmaceutical imports from India, China, and Europe, remain a top three source of our trade deficit.
One action that can reverse the trend Setser talks about above, and reshore some generic drug manufacturing is for the federal government to provide tax credits to American-owned and operated domestic companies that invest in advanced manufacturing of APIs, Gibson said.
MADE IN AMERICA.
CPA is the leading national, bipartisan organization exclusively representing domestic producers and workers across many industries and sectors of the U.S. economy.
TRENDING
CPA Sends Letter To Senate Leaders Schumer and McConnell Opposing Advancement of Recent USITC Nominations
CPA: Liberty Steel Closures Highlight Urgent Need to Address Mexico’s Violations and Steel Import Surge
CPA Applauds Chairman Jason Smith’s Reappointment to Lead House Ways and Means Committee
Senator Blackburn and Ossoff’s De Minimis Bill is Seriously Flawed
JQI Dips Due to Declining Wages in Several Sectors as November Jobs Total Bounces Back from Low October Level
The latest CPA news and updates, delivered every Friday.
WATCH: WE ARE CPA
Get the latest in CPA news, industry analysis, opinion, and updates from Team CPA.
CHECK OUT THE NEWSROOM ➔