Former President Donald Trump, who is currently running in the 2024 presidential primary, announced this week that he would make the reshoring of critical medicine a priority in his administration, should he be reelected to the White House in 2024.
“It’s not just a public health crisis; it is also a national security crisis,” he said. “We will phase in tariffs and import restrictions to bring back essential mediation production in the United States,” he said, adding that reshoring production of drugs on the FDA’s critical medication list will be a top priority. As president, Trump signed an Executive Order on Aug. 6, 2020 instructing the FDA to review and make a list of all essential medications in short supply.
 Trump said he signed Executive Order 13944 in 2020 to begin reshoring drug production, but President Joe Biden reversed the order in 2021. The U.S. has a massive trade deficit in pharmaceuticals, with most branded drugs coming from Europe and generics coming from India and, increasingly, China.
Trump said he signed Executive Order 13944 in 2020 to begin reshoring drug production, but President Joe Biden reversed the order in 2021. The U.S. has a massive trade deficit in pharmaceuticals, with most branded drugs coming from Europe and generics coming from India and, increasingly, China.
Imports Keep Rising
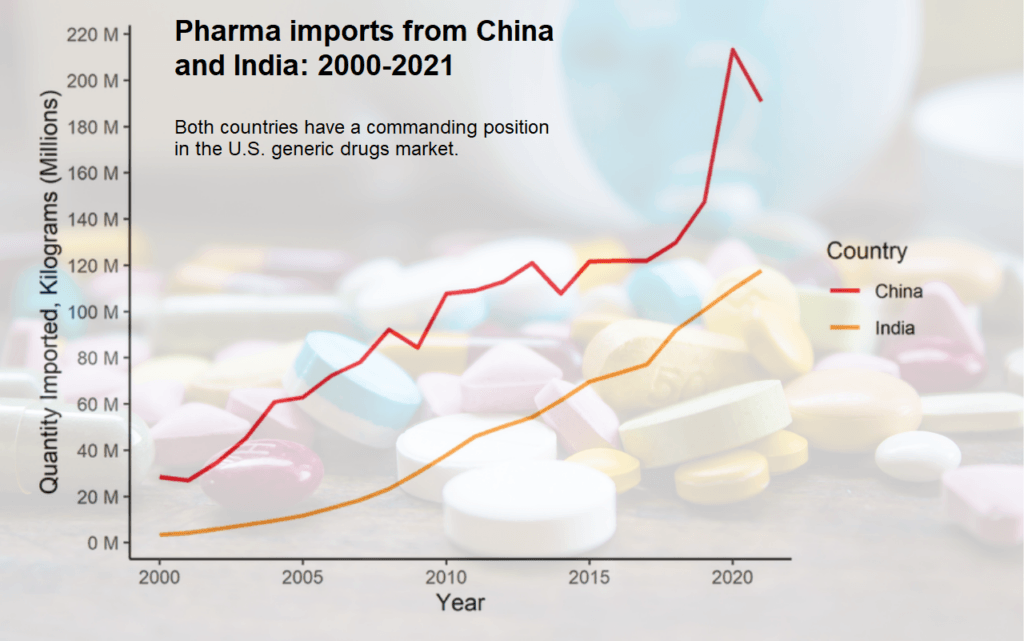
A January 2023 CPA Economics Team report said, “U.S. pharmaceutical imports have skyrocketed in the last ten years, with imports from China, India and Mexico leading the surge. India and China are increasingly the leading U.S. source for generic pharmaceuticals, which account for 90% of all prescriptions written in the U.S.” Additionally, “Growing U.S. dependence on China and India for widely-used generic pharmaceuticals creates serious risks to national security and patient safety. China now accounts for 95 percent of imports of ibuprofen, 91 percent of imports of hydrocortisone, 70 percent of imports of acetaminophen, and 40 to 45 percent of imports of penicillin.”
The FDA often faces a conundrum of allowing for the import of medication from labs that have failed inspection, or risk supply shortages due to placing restrictions on those labs. American patients, and doctors, therefore, have to deal with hunting for alternative medications, change dosages, or risk taking drugs from labs that have received warning letters and import alerts from the FDA. This is now common practice.
Congress has been alerted to this situation. There is interest in making it easier, and financially feasible, to produce generic drugs in the U.S. Congress is also aware of shortages, which was made front page news last year during the Abbott Labs infant formula fiasco.
Congress needs to solve the generic drug crisis by boosting domestic production. Beside tariffs, another solution is production tax credits like those used in the Inflation Reduction Act. Those credits are not for pharmaceutical companies and are only for so-called “clean tech” manufacturers like EV battery companies, EV charging station manufacturers, solar and more in that space. To solve the perennial drug shortage problem, and alleviate the FDA needing decide between drug safety and shortages, similar tax incentives should be used for producers of generic drugs in the U.S.
Today, the United States is dangerously dependent on foreign manufacturers. Of all prescriptions dispensed in the United States, roughly 90 percent are generics and nearly all of these medications are imported from foreign facilities in India or China.
In 2021, CPA produced a 31-page report on pharmaceuticals that explained how a loophole in the 1984 Hatch-Waxman Act led to the offshoring of America’s domestic production of generic pharmaceuticals to China and India, and drug shortages in the United States. The U.S. has had shortages this year of basic drugs like amoxicillin, to more complicated drugs like cisplatin, a chemotherapy drug.
Authors of the Hatch-Waxman Act intended to create competition, increase innovation, and lower prices for U.S. patients. Instead, in many cases, the opposite happened. Foreign manufacturers engaged in a race-to-the-bottom strategy that eliminated U.S. manufacturers from the market. And the offshoring of U.S. production did not lead to more innovation and lower prices, but to the importation of poor quality, unsafe generic drugs, and widespread price manipulation.
This healthcare and national security crisis started long before the Covid‑19 pandemic began. The pandemic revealed to Capitol Hill that America’s dependence on foreign drug manufacturers is a serious problem.
Over the past decade to 2021, pharmaceutical imports have nearly doubled (increased by 89%) and increased by a factor of six in the past twenty years. Pharmaceutical imports have increased substantially more than total imports over the same period, driven by increased U.S. spending on health care, higher drug prices, and the persistent offshoring of drug manufacturing by leading pharmaceutical multinationals. Over the past twenty years, pharmaceuticals went from the ninth most imported good to second only to automotive. Through November 2022, pharmaceutical imports were running 11.5% ahead of their total in 2021, and likely to finish the year at around $196 billion. In light of the seemingly unstoppable growth of healthcare spending in the U.S., pharmaceutical imports are very likely to overtake automobiles within the next few years. Along with growing imports, drug shortages and a lack of regulation over drug safety have emerged as production of pharmaceuticals and the key ingredients necessary to manufacture medicine have become increasingly concentrated in China and India. – “Skyrocketing Pharmaceutical Imports to the U.S. Endanger National Security” by Andrew Heritage, Senior Economist, CPA, January 9, 2023.
Domestic Production Key to Quell Drug Shortages
Some American drug companies are ready to make up for supply constraints.
U.S. Antibiotics, located in Bristol, Tennessee, makes amoxicillin and uses 7 active pharmaceutical ingredients (API) supplied by companies in Europe. But in an op-ed on July 26 published on Real Clear Health, Rosemary Gibson, author of “China Rx” and an advocate for reshoring pharmaceuticals, says the Department of Health and Human Services “is overlooking this domestic producer of a drug that the Food and Drug Administration considers a critical medicine. Why aren’t America’s hospitals and retail pharmacies seeking out a domestic producer to obtain supplies of such a critical drug? Possibly because they’ve become so accustomed to buying billions of dollars’ worth of generic drugs imported into the United States each year,” she wrote.
The status quo is failing. Other presidential candidates will likely agree that more needs to be done to bring the production of critical medicines back to the U.S., including President Biden. This is the perfect issue.
In March, FDA Commissioner Dr. Robert Califf told the House Appropriations Committee that he is “very concerned” about America’s current dependence on other countries for everyday medications—and that reshoring of domestic drug production must be part of the solution.
Skyrocketing Pharmaceutical Imports to the U.S. Endanger National Security