Senate bill 4348, the FDA Safety and Landmark Advancements (FDASLA) Act, had its final committee mark-up on Tuesday, passing with 14 in favor of moving forward and 8 against in a Health, Education, Labor & Pensions Committee business meeting on the bill.
FDASLA is the reauthorization bill, which faced some headwinds recently from the Health, Education, Labor & Pensions Committee’s Ranking Member Richard Burr (R-NC) over the baby formula shortage, a supply chain crisis both parties in the Senate have largely blamed on the FDA.

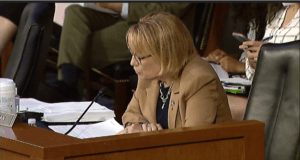
Senator Tina Smith during a Senate markup of amendments that will appear in a number of bills on Tuesday, June 14, 2022.
A total of 25 out of 30 amendments were approved, including Sen. Tina Smith’s (D-MN) amendment that would speed up generic drug approval times. Sen. Maggie Hassan (D-NH) had most of her amendments approved, including one on increased transparency in generic drug applications and three more related to conflicts of interest, which passed unanimously.
The high cost of drugs and the recent baby formula debacle were clearly top of mind during Tuesday’s mark-up meeting. An amendment by Sen. Lisa Murkowski (R-AK) to waive additional requirements for imported formula also passed unanimously. Sen. Mitt Romney’s (R-UT) amendment requiring coordination and consultation between the FDA and companies that have faced food recalls – in this particular case, infant formula – also passed with no opposition.
Click here to see all amendments offered at the Executive Session.
Hassan’s amendment to force the FDA to inform generic drug manufacturers where they got their formula wrong for the inactive ingredients in off-patent drugs passed in Tuesday’s Senate mark-up and will be part of FDASLA.
“The FDA cannot disclose if the concentration of that inactive ingredient is too much or too little. And generic drug manufacturers are then faced with a guessing game,” Hassan said.
Sen. Bernie Sanders (I-VT) made the most noise over drug costs, offering up an amendment that ultimately tabled.
He cited an unnamed poll where he said a majority of people surveyed, over 80%, believed that Congress has not done enough to lower drug prices, adding that U.S. branded drug prices were 1/10th the price of the same product in Canada. He then cited a study by the Journal of the American Medical Association that said the median price for new brand-name drugs has hit $180,000.
“How does this happen? The reality of the pharmaceutical industry charging outrageous prices is because 73% of the FDA budget comes from the industry and not from taxpayers who want lower prices,” Sanders argued. “The agency has an industry regulating it, instead of the other way around. Let’s pass amendments that tell the industry that we regulate them and rather than them regulating the FDA,” he said in his pitch for his re-importation amendment. His amendment would have allowed for online pharmacies to also sell to the U.S.
Sen. Rand Paul (R-KY) said the rest of the world paid less due to price controls. And that multinational pharmaceutical companies, many of them with labs in Europe, then send drugs to the U.S. where prices are not capped and are usually paid for by health insurance companies. He supported the amendment.
Sen. Susan Collins (R-ME) said she knows people from her state of Maine who have doctors in Canada. “They go across the border and get that doctor to write a prescription for them,” but worried that the Sanders amendment was broader than just importing from Canada. “It allows for foreign online pharmacies, which are the source of foreign-made counterfeit drugs,” she said.

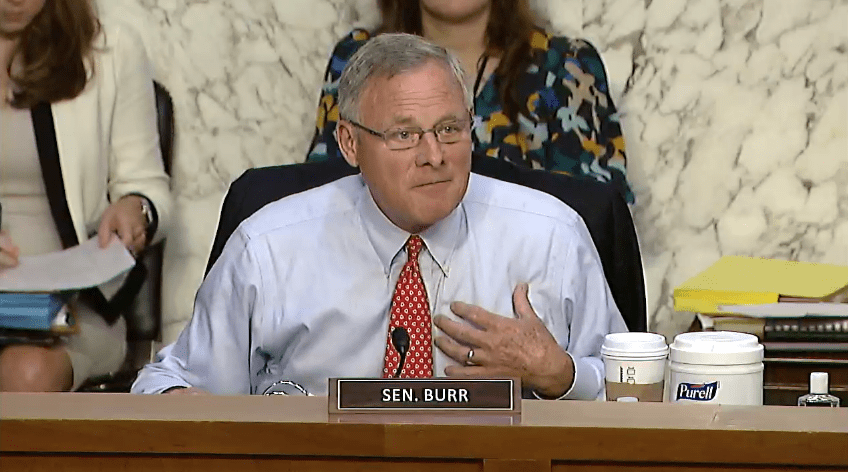
Senator Richard Burr held up to his promise to vote against the current three-year reauthorization bill for the FDA.
Sen. Burr was the vocal opposition, voting against the bill as he promised to just a few weeks ago in a hearing with the FDA Commissioner Robert Califf.
“There is a dark cloud overshadowing this committee today,” Burr said. “The FDA is going to have more authority (with this reauthorization bill), but it is unclear if they deserve our trust.” He called the FDA’s Food Safety Center division a “catastrophe.”
“I’m deeply torn on this legislation (FDASLA). It will bring many reforms to the FDA and encourage the development of the generic drug industry, injecting greater competition into the drug market. (But) we have a long way to go on this bill before it becomes law,” Burr reminded the Committee, adding that while he understands the FDA needs its budget approved for the next three years, he was unsure if “the FDA deserves an ounce of new authority.”