The Senate’s Homeland Security and Government Affairs Committee wants to know what is causing all of these perennial and persistent drug shortages, and whether or not it is wise for the FDA to allow for imports from labs that have received negative reviews following inspections. Most Americans – and that includes doctors – don’t know where their drugs even come from. And the labs, both small and large household names, don’t have to tell you.
“The role of geopolitics and our reliance on foreign products is a major cause of our drug shortages,” said Andrew Shurman, Associate Professor at the University of Michigan Medical School and one of four witnesses in the Committee’s March 22 hearing titled “Drug Shortage Health and National Security Risks: Underlying Causes and Needed Reforms.”
Last year, a GE plant in Shanghai stopped making a chemical contrast used for many radiology tests, threatening nearly half of the U.S. supply of that material. Shortages reached a high of 295 individual drugs at one point last year. Between 2021 and 2022, new drug shortages increased by 30%. According to a 2019 report by Committee Chairman Gary Peters (D-MI), nearly 80% of the manufacturing facilities that produce active pharmaceutical ingredients (API), the key ingredients that give a drug its intended effect, are located outside of the U.S. Most of them come from India and China. In Sen. Peters’ new report, released this week, he revealed that some 90% of generic injectable drugs like anesthesia, for instance, are reliant on China and India, with an equal amount of generic drugs all made overseas.
“We need to diversify where these supplies come from, including increasing production on American soil,” Shuman said. [Testimony] “We should incentivize and/or subsidize companies to make high-quality, critically-needed drugs, even if these are less profitable drugs,” he said.
 In her opening testimony, Erin Fox, Associate Chief Pharmacy Officer for Shared Services at the University of Utah Health, said the most common type of drug in short supply is the generic injectable drugs used in hospitals and clinics. Beyond local anesthetics used to knock people out during surgery, other medications including lidocaine with epinephrine, steroids such as dexamethasone, and chemotherapy agents such as cytarabine and dacarbazine are also often hard to find. This means doctors have to use other drugs if they are available or postpone surgeries and treatments until those drugs become available.
In her opening testimony, Erin Fox, Associate Chief Pharmacy Officer for Shared Services at the University of Utah Health, said the most common type of drug in short supply is the generic injectable drugs used in hospitals and clinics. Beyond local anesthetics used to knock people out during surgery, other medications including lidocaine with epinephrine, steroids such as dexamethasone, and chemotherapy agents such as cytarabine and dacarbazine are also often hard to find. This means doctors have to use other drugs if they are available or postpone surgeries and treatments until those drugs become available.
This is often the main reason why the FDA does not ban imported medication from labs that have failed inspections. Drug manufacturers are not required to provide a public explanation of shortages, as they are not required to provide explanations for voluntary recalls. The only data available on those things are held by the FDA. What the public sees is usually heavily redacted.
“Sixty-two percent of shortages between 2013 and 2017 were due to manufacturing or quality problems,” Fox said. [Testimony] “FDA exercises regulatory flexibility to mitigate the impact of shortages, in some cases allowing (pharmaceuticals) to remain on the market despite containing contaminants and other particles (like glass or hair).”
She said, and most agreed, that the low price of commodity generic drugs, coupled with long-term pricing contracts that cap prices for products even in hot demand and short supply, created a “race to the bottom” environment. “The low prices for some of these products are due to this race to the bottom, which is the result of pressure from hospitals that are paid for most hospital stays with capitated payments and Group Purchasing Organizations that contract on behalf of hospitals. Manufacturers cannot be expected to produce products at a loss,” she said about U.S. generic drugs makers.
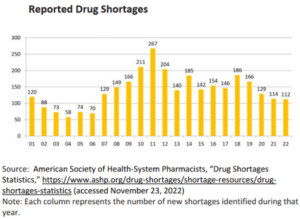 The two and a half hour hearing was one of the first in many years to center on the drug shortages problem. The problem came to the fore during the pandemic when the U.S. struggled to find healthcare materials as simple as face masks, to basic generic medicines common to almost any typical hospital visit.
The two and a half hour hearing was one of the first in many years to center on the drug shortages problem. The problem came to the fore during the pandemic when the U.S. struggled to find healthcare materials as simple as face masks, to basic generic medicines common to almost any typical hospital visit.
One of the witnesses, Vimala Raghavendran, Vice President of Informatics Product Development for US Pharmacopeia, shared her company’s supply chain map. The one-of-a-kind medicine supply map shows where the U.S. has gaping holes in its drug supply.
According to data from US Pharmacopeia’s supply chain map, as of March 2021, nearly 75% of all FDA-registered API manufacturing labs and nearly half all of FDA registered finished dosage manufacturing facilities were located outside of the U.S. Most of what is here is high end, expensive, branded drugs. For the generic drugs that most people take daily, some 87% of FDA-registered labs that make API were located abroad and 63% of FDA-registered finished dosage labs were abroad. By the end of 2021, India accounted for 48% of so-called API Drug Master Files on record. These are the files submitted by labs to the FDA when they intend to supply drug ingredients to other companies for them to make finished product. Europe came in a distant second at 22%, followed by China at 13%. The U.S. accounted for only 10%. Even more interesting: in 2021, India accounted for 63% of all the new Drug Master Files being sent to the FDA. In 2000, it was 20%. They’ve grown four-fold as the global pharmaceutical giants, most of them based in Europe, shifted their manufacturing there. European labs were 49% of new API Drug Master filings in 2000 and were only 7% in 2021. The U.S. is even worse: just 4%. China accounted for 23% of all new API Drug Master Files applied to the FDA in 2021.
Vimala echoed her other witnesses when she said that “Poor FDA inspection outcomes at a facility and products with a history of recalls were correlated with higher likelihoods of shortages. Of the 163 drugs that were in short supply in 2013 and 2017, the FDA found that 62% of them were because of quality issues at those labs.” This likely means voluntary recalls, as FDA warnings from lackluster inspection visits rarely lead to import bans. [Vimala’s testimony]
Hearing Q&A:
 It was a loaded meeting. Everyone had something of interest to say, and the four witnesses were heavy on data and anecdotal evidence. Here are some of those exchanges.
It was a loaded meeting. Everyone had something of interest to say, and the four witnesses were heavy on data and anecdotal evidence. Here are some of those exchanges.
Sen. Gary Peters: How at risk is the U.S. if another pandemic or natural disaster strikes where these drugs are sourced?
Vimala: There is significant reliance on foreign sources when you look at API. When you examine facility and product registration counts, we see that the business is mostly concentrated in India, and then to a lesser extent in Europe and then China. We don’t know where the key starting materials (KSM) are coming from to make that API. We have some anecdotal evidence, but no key understanding of where KSMs are coming from.
Erin: It could look like we have a broad supply chain with five or six labs selling a drug, but if they are all relying on KSM that is solely sourced from one place, well we have no idea about that.
* * *
 Sen. James Lankford (R-OK): What companies are actually bringing manufacturing back here, understanding that we are vulnerable, and are actually doing something about it?
Sen. James Lankford (R-OK): What companies are actually bringing manufacturing back here, understanding that we are vulnerable, and are actually doing something about it?
John Goodman*: It is my understanding that the brand companies that are using overseas suppliers are mainly using Europe or Puerto Rico. The Indian and China markets are the race to the bottom generics market. If all you care about is price, you can go to India and China. That’s where all the generics come from. Mark Cuban said he is building a generic drug plant here in the US. For him to be successful, he is going to have to show that his product is reliable, and efficacious. And he will sell directly to the consumer. Maybe that model will work better.
Lankford: It can’t work worse than the pharmacy middleman model we currently have.
Goodman: I would agree with that.
Vimala: We have the promise of continuous-advanced manufacturing practices. This type of manufacturing technology and processes will help bring medicine production onshore. It might even make sense to do that with critical medicines. It has lower production costs, has a low environmental footprint, but you need to make it easier for manufacturers to enter the market. We need to make it easier for them, especially workforce development. People here don’t know how to work with this equipment.
Sen. Lankford: We were very good at pushing labs to Puerto Rico. We are in trouble. Very few incentives and taxes and the regulatory environment do not help us. When I ask people how quickly we could get the American drug supply out of India and China they tell me decades, not years.
*President, Goodman Institute for Public Policy Research. [Testimony]
* * *
 Sen. Richard Blumenthal (D-CT): Are there still shortages of anesthetics?
Sen. Richard Blumenthal (D-CT): Are there still shortages of anesthetics?
Shuman: Yes.
Sen. Blumenthal: There is something wrong here when anesthetics, used by every hospital in the country, is not responding to demand signals. Why aren’t we seeing more supply responding to all the demand? What’s wrong?
Goodman: If you pay me, I’ll invest in capacity, and I’ll meet your demand. But now, I cannot do that. I can only compete on price and when you can only do that, it is a race to the bottom including a race to the bottom on supply. It’s that simple.
Sen. Blumenthal: If everyone wants more bread, surely bakers and food companies will make more bread to sell to them.
Goodman: Let’s pretend the grocery store owner is like the pharmacist and is compelled to sell you the lowest-priced bread that comes his way. Well, the quality of the bread would go to the floor. Plus the manufacturing process is not reliable.
Sen. Blumenthal: Okay, so if I make satellites and put them into space and it doesn’t go into space, it’s not reliable. No one will buy it.
Goodman: The FDA does not allow me to charge you more based on safety or reliability, I can only compete on price.
Erin: There is no incentive for one company to do a better job than the other. We have no way to know who is a good lab; there is no rating system. People’s lives are at stake when you have to use a lower-quality product because of supply issues.
* * *
 Sen. Maggie Hassan (D-NH): We understand that mostly all of our API for generics is coming from India and China. And relying on China is a national security and public health risk. How can we make it here?
Sen. Maggie Hassan (D-NH): We understand that mostly all of our API for generics is coming from India and China. And relying on China is a national security and public health risk. How can we make it here?
Shuman: I agree, this is a national security concern. I think incentivizing production on American soil is critical. The devil is in the details. If we have crumbling bridges, we fix them. If we don’t have the labs we need, we need incentives for companies to make critical drugs that are expensive and complicated to make, but we can incentivize them to make it here.
* * *
Sen. Jacky Rosen (D-NV): We need to figure out what those incentives are to increase pharmaceutical independence. Last congress I worked with Senator Tim Scott to introduce the bipartisan Made in America Act to create a tax credit for pharmaceutical companies to operate in certain distressed communities. Some of the provisions of that bill were passed into law, but we need to do a better job bringing life-saving drugs here.
Goodman: We have a good understanding of the problem, and the main problem is the price issue and if you are racing to the lowest price, you are going to run into problems with reliability, safety, and face shortages as we see now. You can inspect a U.S. plant anytime anywhere, but not in India. So we are also held to a higher safety standard than those guys are in India.
Shuman: We as doctors have no way of gauging the quality of these drugs.
Erin: Yes, that is true. There is no requirement for any pharmaceutical company to say where the drugs were made. The FDA can’t share that information, legally. Congress can pass a labeling law to make companies disclose where it is made.
Vimala: Only 3% of labels will tell you where the drugs are from. We analyzed this. The finished dose manufacturer that purchases the API of course will know where it comes from. The FDA has certain confidentiality requirements that bind them.
* * *
Sen. Ron Johnson (R-WI) said that he has been hearing about drug shortages from the FDA for the past three years. And, “We have done nothing,” he said. “You spend a billion here, and a billion on the CHIPS Act, and there is nothing for API or generic drugs.”
Sen. Josh Hawley (R-MO) said that China’s foray into the drug market, while a new phenomenon, is helped by its Most Favored Nation trading status. “They have taken control of manufacturing and surely some of this drug market, too. It is time to change course. This overexposure to China is a problem.”
The U.S. faces a trio of risks that lead to drug shortages: geographic concentration, quality failures leading to recalls, and the commodification of generic drugs which have created a “race to the bottom” on price.
Everyone is on some sort of medication or will need generic medication at some point in their lives, either as a temporary treatment or for a hospital visit. Keeping those drugs cheap is important, as is maintaining their efficacy and safety profiles. But the U.S. will struggle to compete on price for generic drugs. And because shortages are such a problem, the exporting labs will still be able to sell into the market here regardless of efficacy and safety.
“When I’m talking to my patients, they don’t care where the drug is coming from really, they only care when they don’t have access to it,” said Shuman. “What we are dealing with is a situation where doctors and patients are handcuffed due to the unpredictability of drug shortages. We are vulnerable as a nation, we are vulnerable as individual patients and doctors to this issue and I hope we can bring supply back to the United States where it would be more reliable.”